Summary
Curing cancer: changing mindsets to open a window of opportunity
Lisa Hutchinsona, Julian Downwardb and John Hickmanc
(a1 Stone House Gardens, Caterham, Surrey, CR3 6AW bThe Francis Crick Institute, 1 Midland Road, London NW1 1ST; c126 bd Pereire, 75017 Paris, France)
When billions of dollars are spent on cancer therapies that may have only a marginal effect on the outcome of disease¹.² or benefit only a fraction of patients, it is time to take stock. So it was, at the recent meeting “The Challenges Preventing Cancer Cure” ³ (full abstracts Programme Workbook). The meeting addressed the question “why is it so hard to turn fundamental knowledge into effective cures and how can we do this better in the future?” The responses to this question and the suggested solutions were diverse and occasionally radical (see Programme Workbook)³. Speakers, including a patient with terminal cancer, were each asked to define two major barriers to progress and to suggest solutions³. Whilst prominent members of the pharmaceutical industry tended to promote conservative strategies, such as the continued pursuit of drug combinations (see abstracts³), others suggested that more transformative changes in approaches to reduce cancer deaths were necessary. The pharmaceutical industry, supported by academia, was considered by some to be an over-dominant socio-economic force in determining the cancer agenda (the blue lines of Figure 1) and that a broader stakeholder involvement is necessary to better foster health care and reduce cancer mortality. Changes of strategy are in the air, with a former head of cancer drug discovery for the “creation of a world without disease”, preventing disease before it happens, especially the investigation of pre-malignant conditions, such as MGUS4. Disruptive forces (“Landscape developments”, Figure 1), not least in health economics and informed patient pressure, will inevitably change current paradigms and mindsets, with major implications for the current players engaged in cancer research and clinical oncology. The meeting attempted to capture where some of these disruptive forces may arise, how there could be a cultural change of direction that might better benefit patients with cancer or to prevent people from presenting with advanced disease in the first place.
What were considered to be the barriers “preventing cancer cure”?
First, there was a universal agreement by participants that the complex biology of cancer – its genetic and immunologic heterogeneity, its biochemical plasticity and adaptability – is the major barrier to attempts to prolong the survival of patients with advanced, metastatic cancer5, 6. There was a consensus that developing the tools for early diagnosis, such as liquid biopsy7, 8, should be a priority. This would require shifts in research funding. However, over-diagnosis of malignancy based on molecular markers, and consequent inappropriate intervention, is a major challenge9. Nevertheless, treatment is desirable before the branching evolution of a cancer generates multiple and different genetic drivers of the disease. Likewise, adjuvant treatment immediately after surgery or radiotherapy, when tumour burden is low, with potentially reduced heterogeneity, was presented as an appropriate approach.
A second set of major barriers to progress emerged at the meeting. These were considered to be human-imposed and were largely cultural and infrastructural. One example was the manner by which the ‘significant’ impact of systemic therapies is often determined using surrogate endpoints in clinical trials, largely sponsored by industry10-12. These may facilitate early registration of new drugs by pharmaceutical companies, but surrogate endpoints, such as progression-free survival (PFS) often do not lead to significant increases in the hard endpoint of extended overall survival10-12. Clinical trial data describing the probability of an increased PFS (and not overall survival) of a few weeks or months, claimed to be “transformative” in disease treatment – aimed at permitting drug registration and marketing – was considered to be questionable. Many participants agreed that the bar is set too low describing what constitutes significant progress in the therapy of advanced metastatic disease13. The drug regulatory agencies, such as the FDA, NICE and EMA, are the means by which elected governments impose the expectations of society on the commercial sector; in the area of cancer there is a perception that they are willing to accept far too minor incremental improvements and are also much too closely connected with the industry that they regulate. Raising the bar would have economic and strategic consequences, initiating the reallocation of resources perturbing the current model, thus leading to better science, less waste and better outcomes.
Whilst the pharmaceutical industry might be considered as the dominant sociotechnical system driving how cancer is treated (Figure 1), the current agenda and culture of academic cancer research is intertwined with industry. Industry, after all, builds its workforce – and aspects of its culture - by recruiting from academic laboratories. Over-reductionist approaches in academia to complex biological problems such as cancer have, despite massive public and charity funding, not delivered proportional increases in health14. Disproportionate academic investment in the “Biomedical Bubble” rather than in a balanced “health” portfolio involving a more diverse community concerned with healthcare was suggested to impact negatively on overall indicators of wellness 15. In addition, the self-inflicted pressures of publication policy in academia, required for career advancement, have been blamed for staggering levels of data fabrication16, 17 (see abstracts) and, in turn, the huge waste of time as other academics, but mainly industry, struggle to repeat data to validate novel potential targets or biomarkers18 of disease. Systemic change is required in academia and can be influenced by the major funders15.
Other human-imposed barriers limiting an impact on patient survival and quality of life were addressed at the meeting (see abstracts online3). Systems of access to and information about current or new treatments available for patients were considered to be sub-optimal or even “broken”19. Geographical and social inequalities of cancer treatment and care, even within the UK, were required to be addressed urgently, as was an assessment of its real value20, 21. It was widely concluded that current drug pricing model was unsustainable 22. Economics could be a major driving force for change, with one speaker questioning whether the “cancer gold-rush” in the pharmaceutical industry, rife with project and clinical trial duplication, will actually see them losing money (see online abstract3). One particularly egregious area of economic wastage was identified as the conduct of large numbers of costly phase III trials with drugs that have recently been approved in one indication on other tumour types and settings where there is only the most marginal evidence for likelihood of success. Moreover, with respect to economic drivers, there was a question as to whether current patent laws, giving drug discovery’s long-term returns for investors, inhibit investment in and the progress of innovative areas such as improved diagnostic technologies where intellectual property is more difficult to claim.
There were thus enough challenges to the current model of cancer medicine at the meeting to suggest that a transformative “window of opportunity” may open soon (Figure 1). Indeed, a goal of the meeting was to alert the cancer research community to that opportunity. What new technologies, business models or behaviours will impose change? This is difficult to predict. What was clear was that diverse stakeholders should play a role in evolving the strategies to reduce the cancer burden. This would include a more assertive role of agencies, such as the FDA and NICE and health technology assessment bodies, responding to informed patient pressure23. There should be greater investment in diagnostics, further developing technologies such as liquid biopsy, as well as the application of artificial intelligence, telemedicine and, not least, there has to be social change.
In a study of how 19,000 people perceive change over the various decades of their lives, most people acknowledged considerable change in their past decade, but significantly underestimated what change there would be in their up-coming decade – the “history illusion”24. The inertia imposed by existing social-technical systems (blue lines of Figure 1) is open to question. History tells us that change will come, for the benefit of the burgeoning population who may succumb to cancer. The meeting at the Francis Crick Institute alerted people to open the window of opportunity (Figure 1) over the next decade.
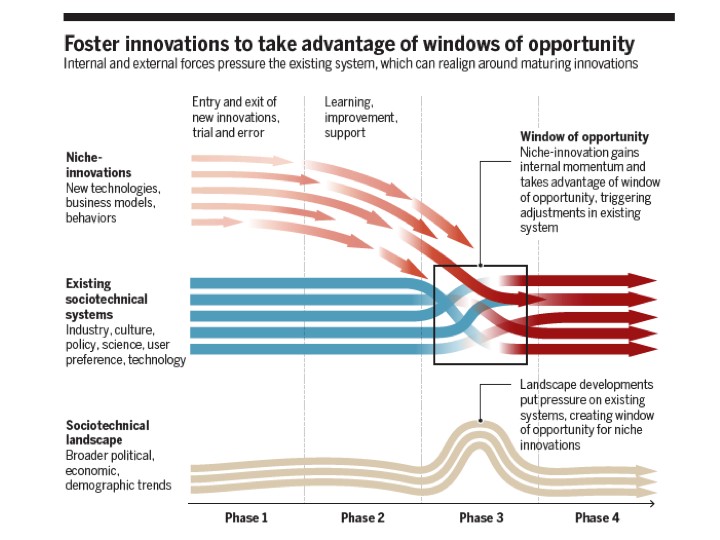
Figure 1. A model of the progress of change to existing socio-technical systems. From: Geels, F. W. et al (2017) Science 357: 1242-1244 (with permission and with thanks to Fred Steward).
References
1. Fojo,T., Mailankody, S. and Lo, A. (2014) JAMA Otolaryngol. Head Neck Surg. 140:12251236.
2. Davis, C., Naci, H., Gurpinar, E., Poplavska, E., Pinto, A. & Aggarwal, A. (2017) BMJ 359: j4530 doi:
10.1136/bmj.j4530
3. Programme Workbook
4. Hait, W. N. & Levine, A. J. (2014) Science Trans. Med. 6: 23.
5. Turajlic, S., Sottoriva, A., Graham, T. & Swanton, C. (2019) Nat. Rev. Genetics doi: 10.1038/s41576-
019-0114-6.
6. Rosenthal R, et al. (2019) Nature 567:479485.
7. Bardelli A, Pantel K. (2017) Cancer Cell. 31:172179.
8. Cohen, J. D. et al (2018) Science. 359:926930.
9. Just, J. https://scienceblog.cancerresearchuk.org/2018/03/06/overdiagnosis-when-finding-cancer-can-do-more-harm-than-good/
10. Booth, C. M. & Eisenhauer, E. A. (2012) J. Clin. Oncol. 30, 1030–1033.
11. Gyawali, B. and Prasad, V. (2019) Ann. Oncol. 30: 361363.
12. Gyawali, B., Hey, S. P. and Kesselheim, A. S. (2019) JAMA Int. Med.
Doi:10.1001/jamaintermed.2019.0462.
13. Ellis, L. M. et al. (2014) J. Clin. Oncol. 32: 12771280.
14. Bowen, A. and Casadevall, A. (2015) Proc. Natl. Acad. Sci. 112: 1133511340.
15. Jones, R. and Wilsdon, J. (2018) (2018) Nesta Report: The Biomedical Bubble https://www.nesta.org.uk/report/biomedical-bubble/
16. Prinz F, Schlange T, Asadullah K. (2011) Nat. Rev. Drug Discov. 10: 712713.
17. Balas E. A. & Ellis L. M. (2017) Nature 543: 40.
18. Byrne, J.A., Grima, N., Capes-Davis, A. & Labbé, C. (2019) Biomarker Insights 14: 112.
19. https://justtreatment.org
20. Murage, P., Crawford, S.M., Bachmann, M. & Jones, A. (2016) Health Place 42: 1118.
21. Jönsson, B. & Sullivan, R. (2019) Mol. Oncol. 13: 636–647.
22. https://www.forbes.com/sites/matthewherper/2015/10/13/four-reasons-drugs-are-expensive-of-which-two-are-false/#5e94beea4c3b
23. Aggarwal, A., Fojo, T., Chamberlain, C., Davis, C. & Sullivan, R. (2017) Ann. Oncol. 28:
1738–1750.
24. Quoidbach, J., Gilbert, D. T. & Wilson, T. D. (2013) Proc. Natl. Acad. Sci. USA 339: 9698.
